AptamiR is developing breakthrough therapies based on the research of Craig Mello and Andrew Fire who won the 2006 Nobel Prize for Physiology or Medicine for the discovery of RNA interference.
Scientific Approach
AptamiR’s distinctive model of virtual, outsourced, and cost-effective drug research and development has resulted in the achievement of several proof of concept milestones within a few years in operations
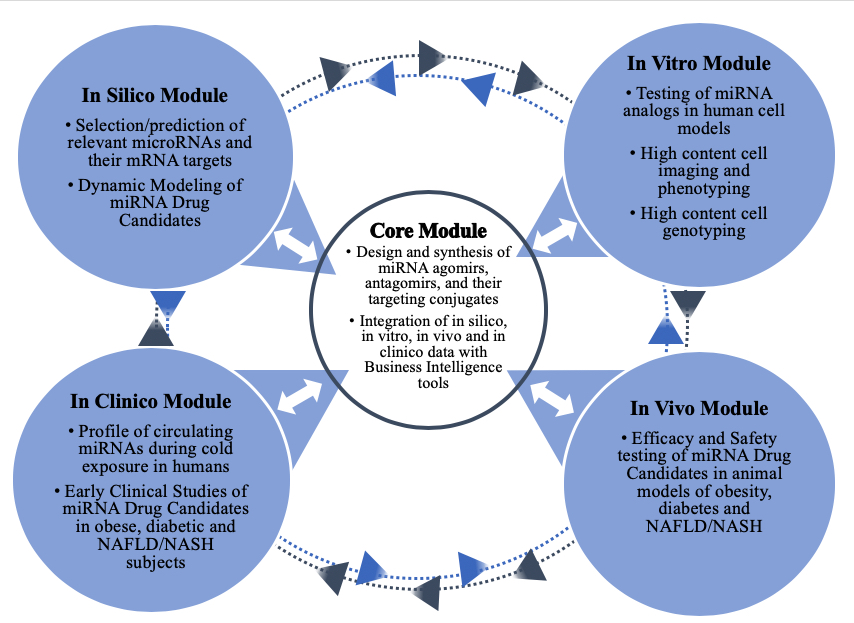
Metabolic Therapeutic Indications: The miRNASH Project
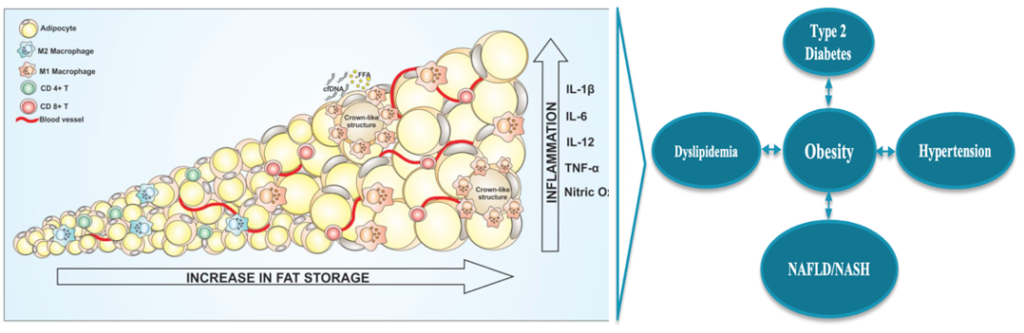
- Human obesity, Type 2 Diabetes Mellitus (T2DM) and Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) are growing worldwide pandemics caused by sedentary lifestyle and excessive consumption of energy-dense foods rich in saturated lipids and carbohydrates.
- Current medical treatments of obesity:
- have poor benefit-to-risk profiles
- fail to reach their long-term goals and
- do not meet patients’ expectations
- There is currently no approved drug for the treatment of MAFLD.
- Obesity and Metabolic Dysfunction Associated Fatty Liver Disease are global pandemics in need of safe, effective and convenient therapies.
- AptamiR’s goal is to develop new therapies that target fat accumulation, inflammation, and necrosis to cure related cardiometabolic disorders (obesity, dyslipidemia, diabetes and metabolic dysfunction-associated fatty liver disease (MAFLD)), without altering brain functions, but improving patients quality of life.
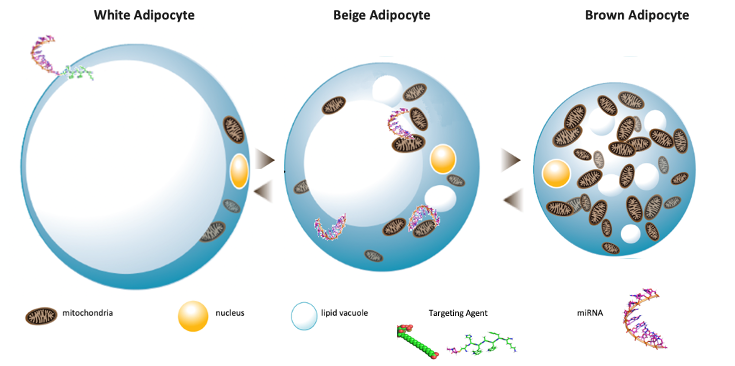
- Our strategy is to develop targeting Oligonucleotide Therapeutics (ONTs) which transform fat-storing cells (white adipocytes) into fat-burning cells (“browning effect”):
- Our End goal is to help patients live longer and healthier lives while reducing healthcare costs.
- Based on an innovative strategy actively targeting subcutaneous fat cells rather than the central nervous system or the intestinal absorption of food, AptamiR is developing microRNA-based therapeutics that enhance lipid oxidation and thermogenesis, turning fat-storing white adipocytes into fat-burning adipocytes (so called brown-like, brite or beige).
- MicroRNA analogs are attractive drug candidates for complex diseases like obesity, diabetes and MAFLD, as they simultaneously modulate the expression of gene networks (One Drug-Multiple Targets concept).
- AptamiR is also developing original drug delivery systems that can specifically supply microRNA analogs to adipose tissues or other particular organs and tissues.
Oncologic Therapeutic Indications: The OvamiR Project
- Ovarian Cancer is the 7th most common type of malignant neoplasms in women and the 8th cause of mortality in them
- More than 60% of patients are diagnosed with advanced diseases (Stages III or IV)
- Worldwide more than 300,000 women are diagnosed with ovarian cancer and more than 200,000 succumb to ovarian cancer every year
- The most common Ovarian Cancers are Epithelial Cancers (90%), among which Serous Ovarian Cancer is the most prevalent (97%)
- High Grade Serous Ovarian Cancer (HGSOC) is the most common and deadliest type of Ovarian Cancer
- Survival to 5 years is only 30% in HGSOC, 18% for patients diagnosed with stage IV tumors
- Ovarian Cancer is a multifactorial disease that cannot be easily controlled by classical therapeutic agents whose Mechanism of Action is one drug-one target or one drug-two/three targets
- Due to its clinical, biological and molecular complexity, ovarian cancer is still considered one of the most difficult tumors to manage and lacks a clear driver mutation
- Presently, debulking cytoreductive surgery represents the gold standard for the treatment of ovarian cancer along with platinum based chemotherapy regimens
- For patients who become platinum resistant, few options are available and efficacy is limited for those regimens
- Our strategy is to develop Oligonucleotide Therapeutics (ONTs) which target relevant miRNAs involved in the proliferation, migration, apoptosis, invasion, metastases, differentiation, Epithelial to Mesenchymal activation and chemoresistance of ovarian cancer
- In vitro testing of Generation 2.5 candidate miRNA agomirs and antagomirs is done in human cells in culture (Epithelial Ovarian Cancer cell lines: SKOV3, SKOV3/CDDP, PA1, CAOV3, SW626, ES-2, HO-8910
Negative Control cell lines: HepG2 (liver), A-549 VIM RFP (lung cancer))
- In vivo testing of selected candidate targeting miRNA agomirs and antagomirs is done in orthotopic and patient-derived tumor xenograft (PDX) mouse models of ovarian cancer
- Clinical studies will be performed in collaboration with:
- The GOG Foundation/GOG Partners/NRG Oncology and the Industry Collaboration Team
- The ENGOT network of ESGO